An ISO 9001 Project Plan will give you an overview of the steps required for achieving certification as well as a better understanding of the scope of the project.
Most ISO 9001 projects take 3 to 12 months.
Although you can certainly speed up the process and do it quicker, most certification bodies wish to see at least 3 months of record history before their audit.
Our ISO Project Plan Templates is proven to work.

Buy a copy of the ISO 9001:2015 standard - this is essential!
Start learning about ISO 9001:
Learn about the Ten ISO 9001 clauses
Generally, Certification Bodies audit small companies for 1 day, once per year. Bigger organizations are audited twice per year. To get information on potential auditors please see:
NOTE: you are audited and "certified" by Certification Bodies; Accreditation Bodies audit and "accredit" the certification bodies.
The purpose of this gap analysis is to identify the areas in your company that require change in order to be compliant.
Our Quality Manual Template includes a 18-page gap analysis template which:
A gap analysis will compare your current systems with the requirements of the standard. Where there is a shortfall, it is called a "gap". This will show the processes:
The gap analysis will give Top Management a steer as to the scope of effort and resources needed to gain certification.
Every sub-clause of ISO 9001 Section 5, Management Responsibility, begins with the phrase, "Top Management shall..."
Top Management commitment is essential. Make sure they understand they must provide evidence of their commitment to developing and implementing the Quality Management System, as well as continually improving its effectiveness.
Unsure they understand they will be interviewed by the Internal Auditor and the Certification Body Auditor.
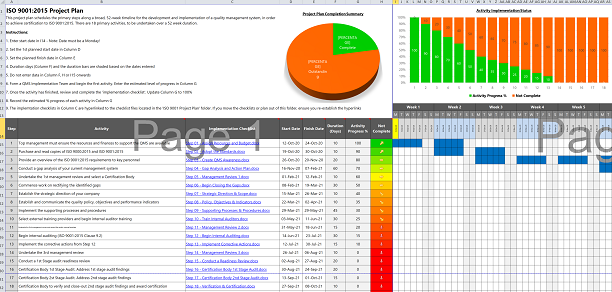
Develop your project plan based on your gap analysis and decide how much or how little documentation you need to demonstrate control.
Our ISO 9001 Project Plan Template is proven to work.
It's important the Quality Policy is defined by Top Management.
The reason you need to define 'quality' is simply that, if you don't know what it is, you'll never know whether or not you are achieving it.
Not knowing where you want to get to also makes it difficult to communicate to other people what is to be achieved and why, let alone to motivate them to act.
Your must define your Quality Objectives. These must reflect the quality policy, be coherent, and align with the overall business objectives, including customer expectations.

Use our Quality Manual Template to help document your Quality Management System (QMS).
View Quality Manual PDF sample.
Use the outputs of your gap analysis to document your processes. These generally include:
Internal Auditors should be people chosen from across the organization that are inquisitive and open-minded.
Most certification bodies require at least three months history between the formal implementation date of the QMS and the certification audit.
Typically, they require that at least one internal audit covering all elements of the QMS are completed and followed by a management review before the certification audit. This enables the company itself to identify problems and to resolve them prior to assessment by the certification body.
The internal auditors need to understand how the clause structure and requirements will affect their audit plans. Instead of auditing by clause, your organization may decide to audit by functional area.
Develop a competence and training schedule for the internal auditors, ensure this is in your project plan.
Learn more about internal auditing
Monitor and measure process performance and start internal audits.
Our Quality Manual Template comes complete with all necessary Forms, including:
Select your Certification Body and agree the Scope of Registration.
Our ISO 9001 Project Plan Template is proven to work.
Make any necessary changes to the QMS and Quality Manual. Most certification bodies wish to see at least 3 months of record history.

The Management Review is your final check to ensure that everyone is happy therefore you should review the business, not just "quality". This vital step is traditionally represented by a minimal, typically annual, senior management review of the QMS.
ISO 9001:2015 requires that the review generates decision on key matters such as process improvement, resource allocation, product improvement driven by customer requirements, and the establishment of new improvement objectives.
Bearing in mind the importance of these sorts of topics, it is best not to hold a separate review, knowing that this sends signals to people in the organization that quality is outside the normal activities of management.
Implement any changes to the Quality Management System that might have arisen from the outputs of previous steps.
The Documentation Audit is a desk-based exercise carried out by auditors either in their own offices or at the company being audited. The audit is restricted to the quality manual and related systems. Its' aim is to ensure that the documentation addresses the elements of ISO 9001. If the auditors identify major gaps in your QMS, there is little point in proceeding with the assessment until these are rectified.
Learn about choosing an ISO consultant.
The Pre-Assessment Audit (optional, also know as the ISO 9001 Pre Accreditation Audit) is a mock audit in preparation for the real thing. A pre-assessment identifies problems and enables the company to benefit from the advice of the auditor on how to eliminate those problems.
You may pay extra, but it is often worthwhile to arrange a pre-assessment visit. Make sure you understand and agree any non-conformances. If not, ask for a second opinion.
Arrange certification date.

This is an on-site audit. It involves a systematic examination of the company's QMS against the ISO 9001 standard.
The emphasis is placed on finding objective evidence that demonstrates it has been implemented effectively and that any procedures are being followed.
The first areas generally examined are management commitment (quality policy and communication), management reviews, corrective actions taken, quality objectives, continual improvement and changes made as the result of the pre-assessment audit.
Make sure you understand and agree any non-conformances. If not, ask for a second opinion.
Our ISO 9001 Project Plan Template is proven to work.
Processes can always be more efficient and effective, even when they're producing conforming products. The aim of a continual improvement program is to increase the odds of satisfying customers by identifying areas needing improvement. After setting improvement objectives, an organization searches for possible solutions, selects and implements the appropriate one and evaluates results to confirm that objectives are met.
ISO 9001 Implementation
Updated: 3rd Feb 2024
Author: Richard Keen

Richard is our Compliance Director, responsible for content & product development.
But most importantly he is ISO's biggest fanboy and a true evangelist of the standards.
Learn more about Richard
Don’t Try to Manage It All Alone!
Our ISO Auditors and Quality Manager Trainers have been in this industry for years, and since 2002 we’ve been providing thousands of small businesses and large corporations with the tools they need to get certified.
Instead of trying to create a project plan and checklist from scratch, use ours.
Before you invest all the hours reinventing the wheel, before you spend countless dollars outsourcing the task — try our templates.
| QMS ISO 9001 |
EMS ISO 14001 |
OH&S ISO 45001 |
|||
ISO 9001 Project Plan and Implementation Checklists |
A complete 18-step project plan for ISO 9001 using MS Excel.
Implementation checklists for each of the 18 steps, MS Word.
|
|
$39 USD |
||
ISO 14001 Project Plan and Implementation Checklists |
A complete 18-step project plan for ISO 14001 using MS Excel.
Implementation checklists for each of the 18 steps, MS Word.
|
|
|
$39 USD |
|
ISO 45001 Project Plan and Implementation Checklists |
A complete 18-step project plan for ISO 45001 using MS Excel.
Implementation checklists for each of the 18 steps, MS Word.
|
|
|
$39 USD |
|
ISO 9001 + ISO 14001 |
A complete 18-step project plan for ISO 9001 and ISO 14001.
Implementation checklists for each of the 18 steps, MS Word.
|
|
$39 USD |
||
ISO 9001 + ISO 45001 |
A complete 18-step project plan for ISO 9001 and ISO 45001 using MS Excel.
Implementation checklists for each of the 18 steps, MS Word.
|
|
$39 USD |
$39 USD |
|
ISO 9001 + ISO 14001 + |ISO 45001 |
An integrated 18-step project plan for
Implementation checklists for each of the 18 steps, MS Word.
|
|
$39 USD |
||
Pay by Credit Card, Debit Card, PayPal or Apple Pay.


|
Please read our Money Back Guarantee. |
Bought by Small Businesses and Large Corporations our templates have been sold online and CD since 2002.
Used by:
The Templates are used by first-timers following our step-by-step, clause-by-clause guidance documents; and experienced Quality Managers wishing to streamline and improve their existing documentation.
The application of our templates is scalable and generic; regardless of the size and type of organization. The elements that form the quality management system are the same.
1. Our customizable templates save you time and money by offering a streamlined process to create your quality documentation
2. They’ve got everything you need in one simple template
3. Proven to work our templates have helped thousands of businesses big and small achieve certification
4. Documents use styles to make reformatting and rebranding a breeze
5. Our templates are generalizable for any industry or sector. The application of our templates is scalable and generic; regardless of the size and type of organization.